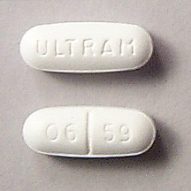
Tramadol: Now a schedule IV controlled drug | Dr. Justine Lee
According to the Drug Enforcement Adminstration (DEA), tramadol, a common pain medication used in both veterinary and human medicine, will become a schedule IV controlled substance effective August 18, 2014. What does this mean to you as a veterinary professional? For veterinarians who handle, stock, or dispense tramadol or tramadol-containing substances, they will be subject to the regulatory controls and administrative, civil, and criminal sanctions applicable to schedule IV controlled...
Read MorePetrem recall of generic sevoflurane
If you use the generic form of sevoflurane called Petrem (made by Piramal Care), pay heed! Piramal Care is issuing a voluntary recall (e.g., not mandated by the FDA) due to one lot not meeting the “acidity/alkalinity specifications set forth in the USP monograph for sevoflurane.” This is a class III recall, meaning that use or exposure of the product is unlikely to cause any adverse medical or health consequences. This affects the following veterinarians, physicians, and...
Read MoreHow new regulations of mouse and rat poison affect your dog
12 d-CON Products Being Canceled and Phased Out The Environmental Protection Agency (EPA) announced on May 30, 2014 that there are new changes to mouse and rat poison (often called “rodenticides” by veterinary professionals). The EPA’s goal of implementing these changes were to reduce access to the rodenticides that pose a poisoning danger to children, pets, and wildlife. While the EPA initiated this mandate in June 2011, Reckitt Benckiser fought the restrictions, suing the...
Read More

Recent Comments