Recent Posts
Recent Comments
- Name *Danielle on My cat has squamous cell carcinoma | Dr. Justine Lee, DACVECC, DABT, Board-Certified Veterinary Specialist
- user-399256 on Pet Hoarders: How many cats are too many? | Dr. Justine Lee
- Aileen on Should you get an automatic pet feeder for your dog or cat?
- Name *PP on How to euthanize a dog with Tylenol… and why you don’t….
- slkdj on Pet Hoarders: How many cats are too many? | Dr. Justine Lee
Archives
- February 2022
- August 2021
- December 2020
- November 2020
- August 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- August 2019
- June 2019
- May 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014

Baxter fluid recall | Dr. Justine Lee
Posted by justinelee in Blog, Recalls, Uncategorized, Veterinary
Deerfield, Ill. – Baxter International Inc. announced today it is voluntarily recalling two lots of intravenous (IV) solutions to the hospital/end user level due to the potential presence of particulate matter. The particulate matter in each case was determined to be an insect and was identified as a result of a customer complaint. The matter was identified prior to patient administration and there have been no adverse events associated with this issue reported to Baxter to date.
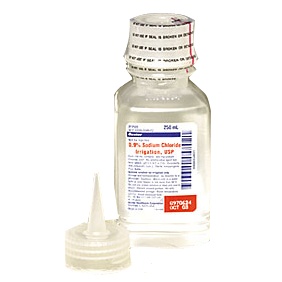
Injecting a product containing particulate matter, in the absence of in-line filtration, may result in blockage of blood vessels, which can result in stroke, heart attack or damage to other organs such as the kidney or liver. There is also the possibility of allergic reactions, local irritation and inflammation in tissues and organs.
This recall affects the following lots:
0.9% Sodium Chloride Injection, USP, 250 mL VIAFLEX Plastic Container is intended for IV use as a source of water and electrolytes and may also be used as a priming solution in hemodialysis procedures. 70% Dextrose Injection (2000 mL) USP is indicated as a source of calories and water for hydration.
The lots being recalled were distributed to customers and distributors in the United States between June 6, 2015 and December 16, 2015. Baxter is directing customers not to use the product from the recalled lots. Recalled product should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7 a.m. and 6 p.m., Central Time. Unaffected lots of product are available for replacement. This recall is not expected to affect current supply and product remains available for current customers.
Customers with questions regarding this recall can call Baxter at 1-800-422-9837, Monday through Friday, between the hours of 8 a.m. and 5 p.m. Central Time, or email Baxter at onebaxter@baxter.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
Adverse reactions or quality problems experienced with the use of these products may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Baxter is voluntarily conducting this recall with the knowledge of the U.S. Food and Drug Administration.
About Baxter
Baxter International Inc. provides a broad portfolio of essential renal and hospital products, including home, acute and in-center dialysis; sterile IV solutions; infusion systems and devices; parenteral nutrition; biosurgery products and anesthetics; and pharmacy automation, software and services. The company’s global footprint and the critical nature of its products and services play a key role in expanding access to healthcare in emerging and developed countries. Baxter’s employees worldwide are building upon the company’s rich heritage of medical breakthroughs to advance the next generation of healthcare innovations that enable patient care.
This release includes forward-looking statements concerning IV solutions, including expectations with regard to its future availability. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter’s most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter’s website. Baxter does not undertake to update its forward-looking statements.
###