Recent Posts
Recent Comments
- Name *Danielle on My cat has squamous cell carcinoma | Dr. Justine Lee, DACVECC, DABT, Board-Certified Veterinary Specialist
- user-399256 on Pet Hoarders: How many cats are too many? | Dr. Justine Lee
- Aileen on Should you get an automatic pet feeder for your dog or cat?
- Name *PP on How to euthanize a dog with Tylenol… and why you don’t….
- slkdj on Pet Hoarders: How many cats are too many? | Dr. Justine Lee
Archives
- February 2022
- August 2021
- December 2020
- November 2020
- August 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- August 2019
- June 2019
- May 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014

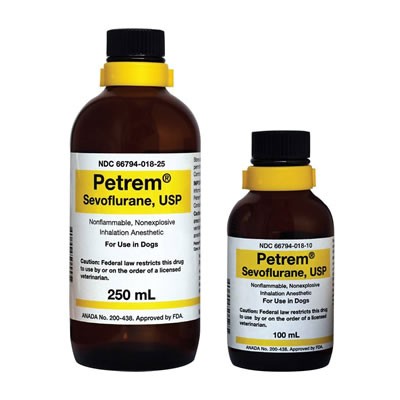
Petrem recall of generic sevoflurane
Posted by justinelee in Blog, Recalls
If you use the generic form of sevoflurane called Petrem (made by Piramal Care), pay heed! Piramal Care is issuing a voluntary recall (e.g., not mandated by the FDA) due to one lot not meeting the “acidity/alkalinity specifications set forth in the USP monograph for sevoflurane.”
This is a class III recall, meaning that use or exposure of the product is unlikely to cause any adverse medical or health consequences.
This affects the following veterinarians, physicians, and distributors in the following states NY, PA, NC, TN, MA, ME, IA, IL, IN, MS, FL, NE, OH, OR, WI, MO, MI, MN, and NJ.
For more information, see the FDA website here.